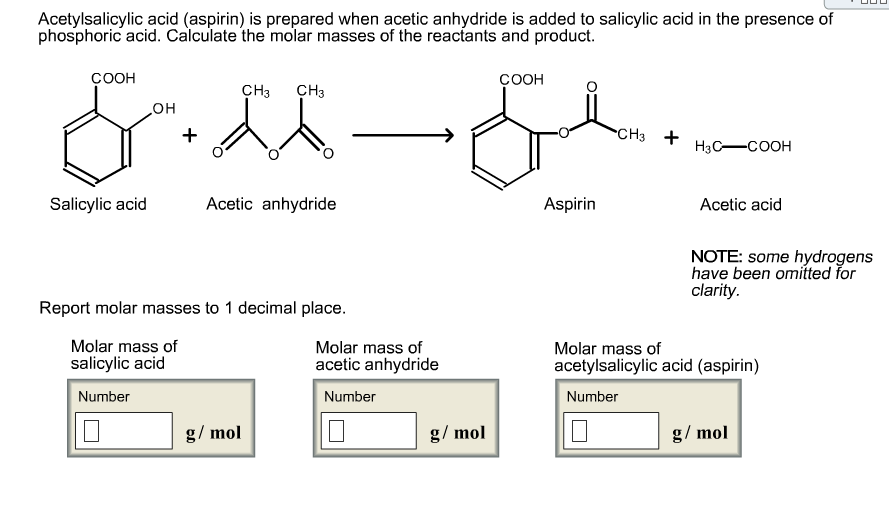
Molar Mass Acetylsalicylic Acid. Its boiling point is 140 ℃. Calculate the molar mass of salicylic acid and acetylsalicylic acid.
Acetylsalicylic acid is a medication that we use to treat pain, fever and inflammation. 38 calculate the molar mass of salicylic acid c7h6o3, which is used to make aspirin; Acetylsalicylic acid (asa), also known as aspirin, which is a commonly used drug for the treatment of pain and fever due to various causes.
12.0107*6 + 1.00794*4 + (15.9994 + 1.00794) + 12.0107 + 15.9994*2 + 1.00794 ›› Percent Composition By Element
Thus, salicylic acid is limiting and a maximum of 0.0305 moles (5.50 g) of acetylsalicylic acid can be produced in the reaction (theoretical yield), based on a 1:1 molar ratio of salicylic acid to acetylsalicylic Based on the number of grams of aspirin that you synthesized, calculate the number of moles of aspirin that you synthesized. 3 rows enter a chemical formula to calculate its molar mass and elemental composition:
1 Mole Acetylsalicylic Acid Has A Mass Of 180G So W Moles Has A Mass Of (180/1) X W Grams You Then Have To Convert Grams Into Grains (1 Gram = 15.4323584 Grains)
Calculate the molar mass of salicylic acid and acetylsalicylic acid. Use the following molar masses for your calculation: Its boiling point is 140 ℃.
The Empirical Formula Of Aspirin= C9H8O4.
Enter a chemical formula to calculate its molar mass and elemental composition: The common name of this compound is aspirin, the medication that we use in our day to day life. Molar mass of (aspirin)c9h8o4 is 180.1574 g/mol convert between (aspirin)c9h8o4 weight and moles
Convert Grams Salicylic Acid To Moles Or Moles Salicylic Acid To Grams.
What is the molar mass of aspirin c9h8o4? The molecular formula of acetylsalicylic acid (aspirin), one of the most commonly used pain relievers, is c 9 h 8 o 4. First of all write the molar masses of gases, molar mass of cl₂ = 71 g/mol molar mass of ethane = 30 g/mol according to graham's law of diffusion, = = = = 1.53 result:
O Knowing The Mass Of The Aspirin Tablet And Given The Molar Mass Of Acetylsalicylic Acid As 180 G/Mol, Calculate The Percent Acetylsalicylic Acid And The Percent Binder In The Aspirin Tablet.
Acetylsalicylic acid (asa), also known as aspirin, which is a commonly used drug for the treatment of pain and fever due to various causes. =2.22!10!!mol c 9h 8o 4 the concentration of the acetylsalicylic acid stock solution then is: Acetylsalicylic acid is a medication that we use to treat pain, fever and inflammation.
Related Posts
- Ch3Ch2Oh MassCh3Ch2Oh Mass. Masa molar of ch3ch2oh masa molar of ch3ch2oh is 46.0684 g/mol ¡eh! Copy sheet of paper on top of another sheet.Solved 2 HaC CH Aceton ...
- Mass Of StyreneMass Of Styrene. The mass reaction is carried out in the presence of solvent. Styrene () is an organic compound with the chemical formula c 6 h 5 ch= ...
- K2Co3 Molecular MassK2Co3 Molecular Mass. Molar mass of k2co3 (aq) is 138.2055 g/mol forget 2020. Molecular weight of k2co3 or grams this compound is also known as potas ...
- Molar Weight Of SucroseMolar Weight Of Sucrose. If there is no subscript, it means one atom is present. Sucrose c12h22o11 molar mass, molecular weight.PPT 1 What is the mol ...
- Conjugate Acid Of Honh2Conjugate Acid Of Honh2. Why is ch3nh3cl an acid in water? The overall salt does not donate protons, the ch3nh3+ ion does (to form h3o+) when the sal ...
- Molar Mass Br2Molar Mass Br2. What is the molar mass of p4? Track your food intake, exercise, sleep and meditation for free.Br2 Molar Mass from divinewsmedia.com👍 ...
- Kno2 Molar MassKno2 Molar Mass. A strong oxidizer used especially in making gunpowder, as a fertilizer, and in medicine. What is the molar mass of kno2?Solved Chemi ...
