Noble Gas Configuration For Strontium. Iodine accepts one electron to. Is noble gas configuration the same as electron configuration?
Strontium tends to form a monatomic ion… | bartleby strontium tends to form a monatomic ion which has the electronic configuration of a noble gas. The noble gas configuration of this element is [kr] 5s2, with [kr] representing the electron configuration of krypton. Its ground state electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1.
On The Other Hand Neon, The Noble Gas, Immediately Preceding It In The Periodic Table, Requires 2081 Kj/Mol Or 21.56 Ev/Atom.
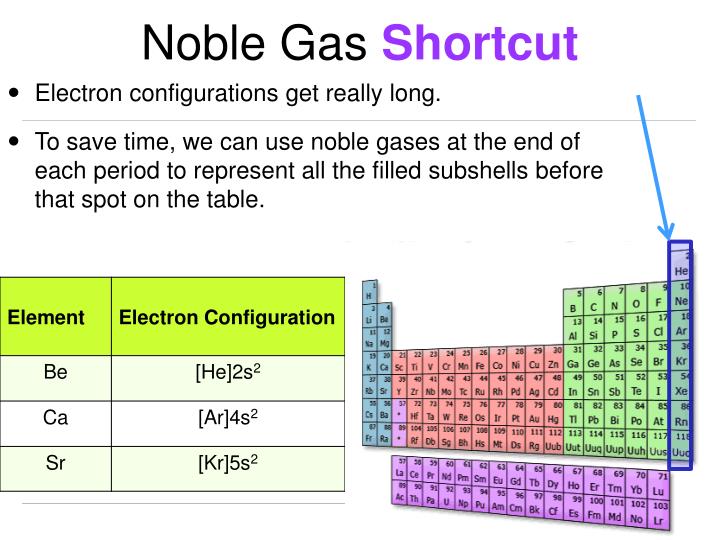
The noble gas notation starts for elements after helium. Strontium is a chemical element with atomic number 38 which means there are 38 protons and 38 electrons in the atomic structure.the chemical symbol for strontium is sr. The noble gas configuration of this element is [kr] 5s2, with [kr] representing the electron configuration of krypton.
Doc Brown's Advanced A Level Chemistry Inorganic Chemistry Periodic Table Revisi.
Strontium will have a noble gas electron configuration similar to that of krypton. The electron configuration for strontium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s2, according to the jefferson lab website. The electron configuration for strontium is 1s2 2s2 2p6 3s2 3p6 3d10.
Describes The Use Of Noble Gas Symbols In Writing Electron Configurations.
The noble gas configuration of this element is [kr] 5s2, with [kr] representing the electron configuration of krypton. Potassium has nineteen electrons, one more than the noble gas argon, so its configuration could be written as [ar]4s1. Strontium tends to form a monatomic ion… | bartleby strontium tends to form a monatomic ion which has the electronic configuration of a noble gas.
The Noble Gas Configuration Of This Element Is [Kr] 5S2, With [Kr] Representing The Electron Configuration Of Krypton.
Electron configuration of strontium is [kr] 5s2. The noble gas configuration of this element is [kr] 5s2, with [kr] representing the electron configuration of krypton. The noble gas shorthand notation is:
Strontium Has An Atomic Number Of.
Strontium has an atomic number of 38, which means it has 38 protons. Click create assignment to assign this modality to your lms. For example, sodium requires only 496 kj/mol or 5.14 ev/atom to ionize it.
Related Posts
- Write The Complete Electron Configuration For Gallium Using The Periodic TableWrite The Complete Electron Configuration For Gallium Using The Periodic Table. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2. For example, 1s2 ...
- Lithium Electron ConfigurationLithium Electron Configuration. In this video we will write the electron configuration for li+, the lithium ion. Combined authors * authors equivalen ...
- Ground State Electron Configuration Of W 3Ground State Electron Configuration Of W 3. Electron configuration of beryllium (be) [he] 2s 2: Removing 3 electrons would give us a total of 71 elec ...
- Ground State Electron Configuration For SiliconGround State Electron Configuration For Silicon. What is the electron configuration of silicon in excited state? For example, [ar]4s23d8 would be ent ...
- Full Electron Configuration For SilverFull Electron Configuration For Silver. Causey shows you step by step how to write the electron configuration of the silver cation (ag+). Therefore, ...
- Electron Configuration Of CobaltElectron Configuration Of Cobalt. Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of t ...
- Mg Ion Electron ConfigurationMg Ion Electron Configuration. Tof mass spectroscopy & electron configuration questions calculate the number of neutrons in the isotope of x whic ...



