Lewis Structure For Propane. Used to make other chemicals. 6 rows propane (c3h8) lewis structure contains three carbon atoms bonding with eight hydrogen atoms,.
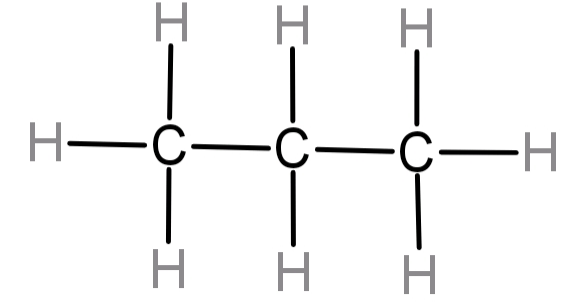
They follow the duet rule (2 electrons). Whenever we see the ending, ane, we know that we're going to have carbons and hydrogens single bonded. Find an answer to your question lewis structure for propane mulenga98 mulenga98 09/26/2018 chemistry middle school lewis structure for propane 1 see answer mulenga98 is waiting for your help.
Here Are A Number Of Highest Rated Propane Line Structure Pictures On Internet.
The exception, of course, being the hydrogen's. The propane chemical formula is c 3 h 8 and is extended formula is ch 3 ch 2 ch 2. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule.
Populate The Electrons Into The Mo Diagram.
Add your answer and earn points. For butane, we have a total of 26 valence electrons. See the big list of lewis structures.
Draw A Lewis Structure Of Ethane.
This is the c4h10 lewis structure: The number of hydrogen bond acceptors and the number of hydrogen bond donors equals to zero. We identified it from reliable source.
The Molecule Is Formed By A Chain Of Three Carbon Atoms Which Are Bound To 3 Or 2 Hydrogen Atoms In Order To Complete The 4 Bonds Required To Complete The Octet Of Lewis Structure.
Propene is composed of 3 carbon atoms and 6 hydrogen atoms. Used to make other chemicals. See the answer see the answer done loading.
C 3 H 8 Uses (Propane) Propane Is Used In Food Additives.
Find an answer to your question lewis structure for propane mulenga98 mulenga98 09/26/2018 chemistry middle school lewis structure for propane 1 see answer mulenga98 is waiting for your help. Add in the missing mo cartoons for the different levels. Draw a lewis structure of propane.